Introduction: CLL/SLL patients who failed BTKi and are treated with BCL2i represent a growing population with few standard-of-care treatment options after discontinuing both therapies. Patients who fail both BTKi and BCL2i have poor prognosis, with a median overall survival (OS) of 3.6 months (Lew et al., Blood Advances, 2021). However, real-world evidence for these patients is limited. This study described the characteristics, treatment patterns, and outcomes of CLL/SLL patients who were relapsed or were resistant/refractory/intolerant (R/R/R/I) to BTKi and who were exposed to BCL2i. Among patients who discontinued BCL2i, responses to the treatment immediately after BCL2i were reported.
Methods: This retrospective chart review study evaluated Dana-Farber Cancer Institute patients with CLL/SLL (≥18 years old) who were R/R/R/I to BTKi and received BCL2i (in any order). Abstraction took place from 12/2021 to 07/2023. Patients were required to have records available from 6 months before to 12 months after first BTKi initiation. Patients who received non-hormonal antineoplastic therapy for other primary malignancies in the period between CLL/SLL diagnosis and receipt of their first BTKi treatment were excluded. Demographic and clinical characteristics at or before BCL2i initiation were described. Lines of treatment (LOTs) and responses to BCL2i were summarized. Time to treatment discontinuation, real-world PFS (rwPFS) and OS were estimated by Kaplan-Meier analysis. Patients were considered doubly refractory if they were R/R/R/I to BTKi and BCL2i. Overall response rate to first treatment after BTKi and BCL2i was summarized among doubly refractory patients.
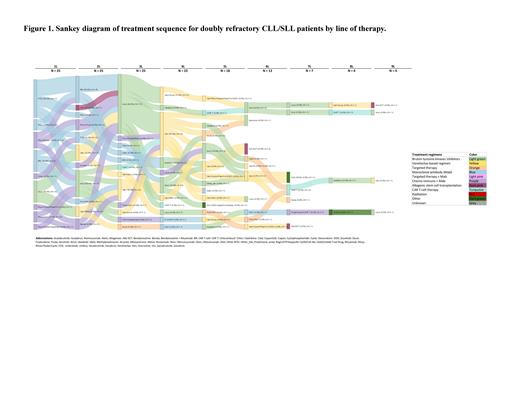
Results: Among 104 patients who were R/R/R/I to BTKi, 61 (58.7%) received a BCL2i. The patients exposed to both BTKi and BCL2i were observed for a median (interquartile range [IQR]) time of 2.6 (1.1,4.4) years after BCL2i initiation. 57 (93.4%) patients received BCL2i after BTKi and 4 (6.6%) received BCL2i before BTKi. BCL2i was most commonly (23 [37.7%] patients exposed to BCL2i) received in the fourth LOT and all patients received their first BCL2i after their first LOT. Median (IQR) age at BCL2i initiation was 68.4 (63.3-74.4) years. Among patients who were assessed for cytogenetic abnormalities (n=58), the most common abnormalities were del(13q) (55.2%) and del(17p) (36.2%). 36 (61.0%) patients had TP53 mutation. 45 (73.8%) patients discontinued their first BCL2i treatment. IGHV mutation was assessed for 49 patients and 8 (16.3%) had mutation rates ≥ 2%. Median (IQR) time to BCL2i discontinuation for patients receiving BCL2i was 1.5 (0.4, 3.1) years. 40 (76.9%) of the 52 patients with a known first response to BCL2i achieved clinician-assessed complete (CR) or partial response (PR) at first assessment. 25 (41.0%) patients were R/R/R/I to BCL2i at the time of abstraction. Of them, 21 (84.0%) progressed, 7 (28.0%) developed Richter transformation (RT) and 11 (44.0%) died. Primary cause of death was available for 8 (72.7%) patients and, of these, 5 (62.5%) died due to disease progression, 2 (25.0%) died due to a secondary malignancy, and 1 (12.5%) died from unspecified causes. Among doubly refractory patients, median (95% CI) rwPFS was 1.6 (0.7, 2.4) years and median OS was 3.8 (2.3, not reached) years from the initiation of the latter of BTKi and BCL2i treatment. Among doubly refractory patients, 18 (72.0%) received an additional LOT after the latter of BTKi and BCL2i. The therapies received in the first line after BTKi and BCL2i were allogeneic HSCT (4 patients), duvelisib (3 patients), oral CDK9i (2 patients), and 1 patient each with CAR-T, anti-CD20 bispecific antibody, alemtuzumab, vecabrutinib, zanubrutinib; and for patients with RT: venetoclax plus rituximab with anthracycline chemotherapy (2 patients), PI3Ki/PD-1 (1 patient), duvelisib (1 patient) ( Figure 1). Response to the additional LOT was known for 17 patients and unknown for 1 patient. Clinician-assessed CR or PR to treatment immediately after BCL2i and BTKi was achieved for 4 (23.5%) of these patients at first assessment.
Conclusions: Patients with CLL/SLL who were failed by BTKi and BCL2i treatment have poor prognosis. Overall response rates to treatment immediately after BTKi and BCL2i treatment are low among doubly refractory patients. More effective treatments are needed to address the unmet therapeutic needs of CLL/SLL patients who are refractory to both BTKi and BCL2i.
Disclosures
Huynh:Genmab: Research Funding; Merck & Co Inc: Research Funding; Novartis: Research Funding; Takeda Oncology: Research Funding; Apellis Pharmaceuticals: Research Funding. Yang:Merck & Co., Inc.: Current Employment. Zanardo:Sun Pharmaceuticals Ltd.: Research Funding; Novartis AG: Research Funding; Pfizer Inc: Research Funding; AbbVie Inc: Research Funding; Takeda Pharmaceutical Company: Research Funding; United Therapeutics Co.: Research Funding; Bristol Myers Squibb: Research Funding; Merck & Co Inc: Research Funding. Matay:Merck & Co., Inc.: Research Funding. Pinaire:Merck & Co., Inc.: Research Funding. Farooqui:Merck & Co., Inc.: Current Employment, Current equity holder in publicly-traded company. De Nigris:Merck & Co., Inc.: Current equity holder in publicly-traded company; Merck Sharp & Dohme LLC (a subsidiary of Merck & Co): Current Employment. Gandra:Merck & Co., Inc.: Current Employment, Current equity holder in publicly-traded company. Sarpong:Merck & Co., Inc.: Current Employment, Current equity holder in publicly-traded company. Duh:GSK: Research Funding; Humacyte: Research Funding; Merck & Co., Inc.: Research Funding; Novartis Pharmaceuticals Corporation: Research Funding; Pfizer: Research Funding; SeaGen: Research Funding; Genmab: Research Funding; Takeda Pharmaceuticals USA, Inc.: Research Funding; Blueprint Medicine: Research Funding; Apellis Pharmaceuticals: Research Funding; AstraZeneca: Research Funding; Ayala: Research Funding. Brown:Gilead: Research Funding; Alloplex Biotherapeutics: Consultancy; Genentech/Roche: Consultancy; MEI Pharma: Research Funding; Merck: Consultancy; Loxo/Lilly: Consultancy, Research Funding; Kite: Consultancy; iOnctura: Consultancy, Research Funding; Hutchmed: Consultancy; Grifols Worldwide Operations: Consultancy; TG Therapeutics: Research Funding; BeiGene: Consultancy, Research Funding; Abbvie: Consultancy; Numab Therapeutics: Consultancy; SecuraBio: Research Funding; Pharmacyclics: Consultancy; Pfizer: Consultancy; Acerta/AstraZeneca: Consultancy. Davids:Eli Lilly: Consultancy; Genentech: Consultancy, Research Funding; Janssen: Consultancy; Merck: Consultancy; Mingsight Pharmaceuticals: Consultancy; Research to Practice: Consultancy; Secura Bio: Consultancy; TG Therapeutics: Consultancy, Research Funding; Takeda: Consultancy; Novartis: Research Funding; Surface Oncology: Research Funding; MEI Pharma: Research Funding; ONO Pharmaceuticals: Consultancy; Adaptive Biosciences: Consultancy; Curio Science: Consultancy; BMS: Consultancy; BeiGene: Consultancy; Aptitude Health: Consultancy; Ascentage Pharma: Consultancy, Research Funding; AstraZeneca: Consultancy, Research Funding; AbbVie: Consultancy, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal